Electronic Data Capture System
Experience the future of healthcare data management with our web-based, GCP-compliant EDC system.
This state-of-the-art system is used to remotely collect and manage patient data in registries, observational studies, and AMG trials, providing a fully digital process that manages sensitive data efficiently and securely.
Our EDC system offers a wide range of functions for efficient and seamless data management and analysis.
eCRF - Flexibel Form Layout
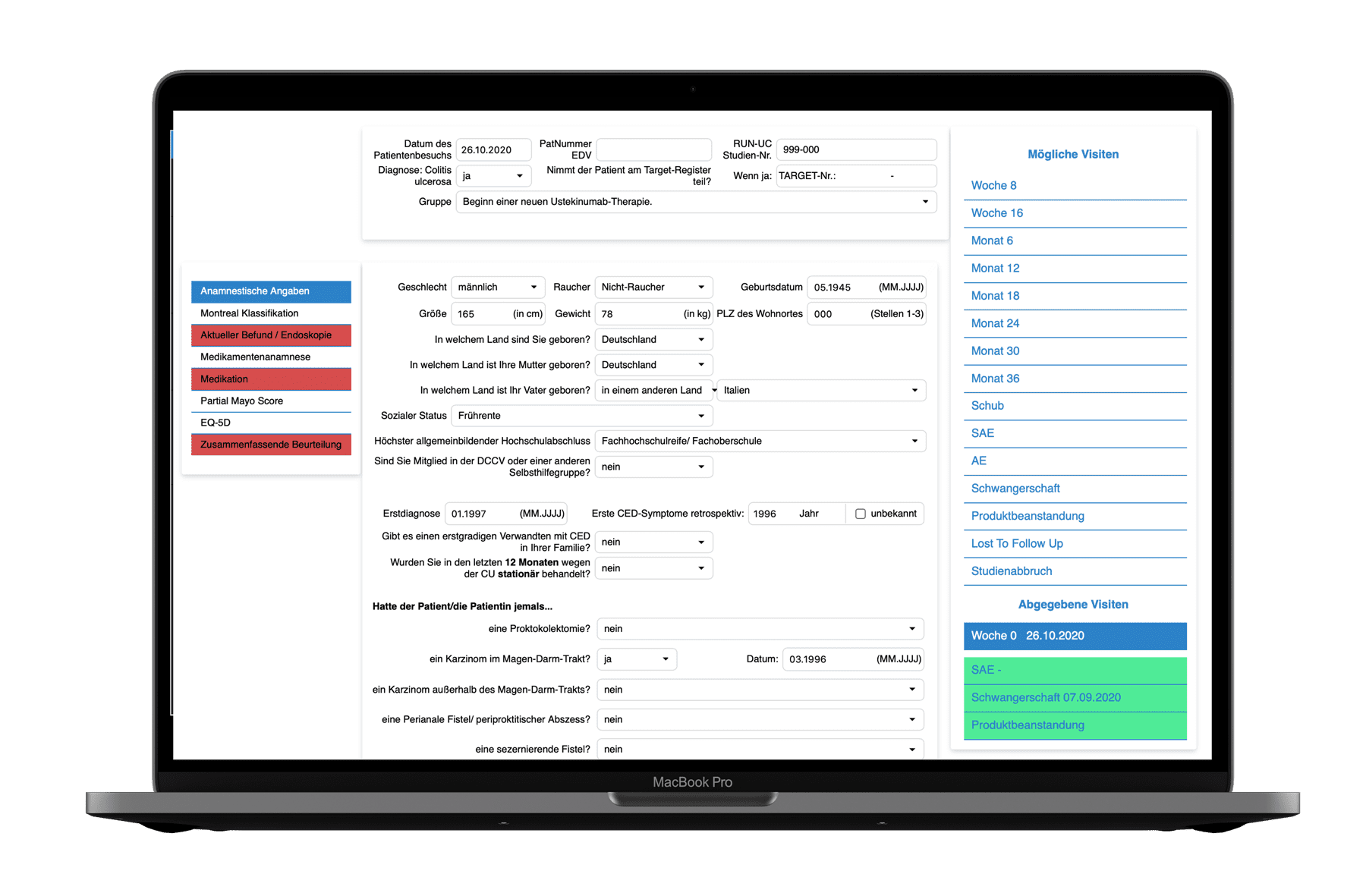
The easy-to-use graphical interface allows you to create and flexibly design any number of eCRFs (electronic case report forms) using drag and drop elements.
Query Layout
Create any number of queries with the EDC system, which can also be output as free and customizable text.
Event-Tracking & Study Status
The visual display of the study or documentation status per patient provides an overview of the completeness of the documentation and thus facilitates an overview of open or answered queries.
Reports & Data Export
With our EDC system, you can quickly export study data from individual patient forms as a PDF. In addition, you can export all study data at any time for import into SAS and other standard formats without major transformation.
Choose from over 20 modules to design your study.
Audittrails
The complete audit trail of user and change information can be output in an easy-to-view format.
Interdisciplinary Concepts
A user can be given access to multiple studies under the same username.
Integration of Coding Tools
The integration of coding tools such as ICD or TNM classification is supported.
Double Data Entry
Data can be reconciled in the eCRF in the event of duplicate data entry from paper CRFs.
Patient Reported Outcome
A web-based tool for Patient Reported Outcomes can be integrated.
Source Data Verification
It is possible to document source data verification for monitoring functions.
Pseudonymization
An external pseudonymization service can be integrated into eCRF.
Randomization
eCRF has a randomization tool and is extensible.
Mailing
Automated emails can be sent to defined groups of people when user-defined conditions are met.
Integration of Images
An image integration module (MRI, etc.) is included in the EDC system.
Payment Management
Various payment management features are supported.
Security
Our hosting services are located in German or European datacenters.

Studienkoffer
In addition to the customized EDC solution, we also offer a study laptop for conducting studies.
This allows you to transfer raw data and image data from peripheral devices to the study. The data is transferred to the study database via the laptop. The software is also available as a stand-alone solution.
Schneller, kostengünstiger & zuverlässig - Dank eCRF, eCRO/ePRO & mehr!
Entdecken Sie unser innovatives EDC System für Klinische Studien!
Vereinfachen Sie Ihre Studienprozesse, verbessern Sie die Datengenauigkeit und sparen Sie wertvolle Zeit. Holen Sie sich noch heute Ihr EDC-System und treiben Sie Ihre Forschung auf die nächste Stufe!
Faster, Cost-Effective & Reliable with eCRF, eCRO/ePRO & More!
Discover our innovative EDC system for clinical trials!
Simplify your study processes, improve data accuracy, and save valuable time. Get your EDC system today and take your research to the next level!

